Structure, Mechanism and Evolution of the
Nuclear Pore Complex
Our lab focuses on aspects of the nuclear information pathway; how macromolecular complexes dynamically interact control the passage and transport of genetic information from intranuclear DNA via RNA through the nuclear pore complexes (NPCs). As the sole mediator of nucleocytoplasmic exchange across the nuclear envelope that encloses eukaryotic DNA, NPCs thus define the contents of the nucleus. The pivotal role of the NPC in controlling communication between the genetic material and the rest of the cell is reflected in the many oncogenic and developmental defects directly associated with alterations in nucleocytoplasmic transport. Nucleocytoplasmic transport depends on the interplay between transport cargoes (which carry targeting sequences that direct the cargos to the nucleus (NLSs) or cytoplasm (NESs)), their cognate soluble transport factors (many termed karyopherins, or kaps), and NPCs.
Rout & Aitchison 2001 (PDF)
As a full understanding of how the NPC mediates transport is needed to discern the nature of these defects, we have taken a comprehensive approach to defining the functional architecture of the NPC in the model eukaryote Saccharomyces (yeast). We have identified all the yeast NPC proteins (nups) and plotted their approximate relative positions, which has allowed us to propose a new mechanism for nuclear transport.
Proteomic data can be used to determine the architectures of macromolecular assemblies. The process involves the collection of sufficient and diverse high-quality data, translation of these data into spatial restraints, and an optimization that uses the restraints to generate an ensemble of structures consistent with the data. Analysis of the ensemble produces a detailed architectural map of the assembly. Thus, we have determined the position, shape and stoichiometry of each nup, and have systematically isolated nup subcomplexes and analyzed their composition by mass spectrometry in order to determine the network of interactions they make. Together, this wealth of information represents thousands of spatial restraints, allowing us to to localize the NPC’s 456 constituent proteins.
25_2000_JCB_Rout
Rout et al., 2003 (PDF)

The nuclear transport cycle
An import-bound Kap binds to its NLS-bearing cargo in the cytoplasm and transits the NPC by the process of virtual gating. On the nucleoplasmic side, RanGTP binds to the Kap, causing a conformational change which releases the cargo. On the other hand, exporting Kaps bind their cargoes in the presence of RanGTP. The resulting nuclear complexes pass the NPC through the same mechanism of virtual gating. On the cytoplasmic side, RanGTP hydrolysis is stimulated by the cytoplasmically-disposed RanGAP resulting in the release of cargo. RanGDP is then recycled to the nucleoplasm by NTF2 and it is reloaded with GTP to begin another cycle.
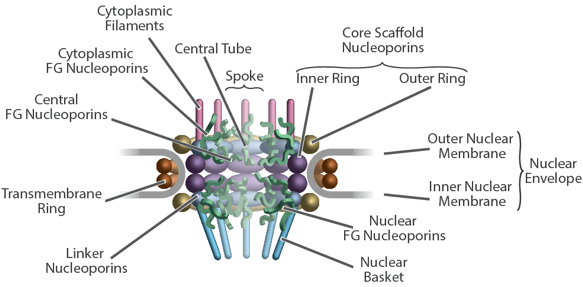
Diagram of NPC and associated transport factors
Despite the central role of NPCs as gatekeepers of RNA and protein transport between the cytoplasm and nucleoplasm, their large size and dynamic nature have impeded a full structural and functional elucidation. As a full understanding of how the NPC mediates transport is needed to discern the nature of these defects, we have taken a comprehensive approach to defining the functional architecture of the NPC in the model eukaryote Saccharomyces (yeast).
Proteomic and interactomic data can be used to determine the architectures of macromolecular assemblies. The process involves the collection of sufficient and diverse high-quality data, translation of these data into spatial restraints, and an optimization that uses the restraints to generate an ensemble of structures consistent with the data. Analysis of the ensemble produces a detailed architectural map of the assembly. Thus, we have determined the position, shape and stoichiometry of each nup, and have systematically isolated nup subcomplexes and analyzed their composition by mass spectrometry in order to determine the network of interactions they make. Together, this wealth of information represents thousands of spatial restraints, allowing us to localize the NPC’s 456 constituent proteins in increasingly detailed maps of its structure.
The structure reveals the NPC’s functional elements in unprecedented detail, providing some striking and sometimes surprising insights. The NPC is surprisingly modular, consisting of only 30 proteins of the nucleoporin family (Nups). The map supports our hypothesis that the NPC is derived from an ancient membrane-coating complex (which we term the protocoatomer), related to the clathrin/adaptin and COP complexes, although the exact arrangement of these coatomer-like complexes in the NPC suggests that the nucleus in fact arrived relatively late in the evolution of eukaryotes.
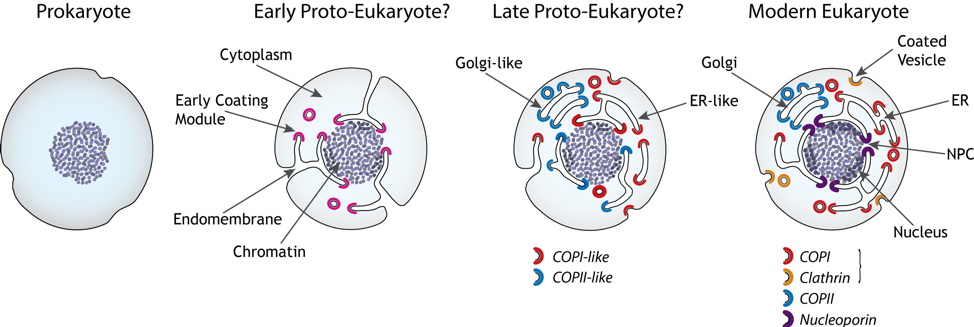
Potential path of evolution of eukaryotes and the formation of membrane coating complexes, NPCs and the nucleus.
Three-dimensional structure of the yeast NPC:
click to enlarge:
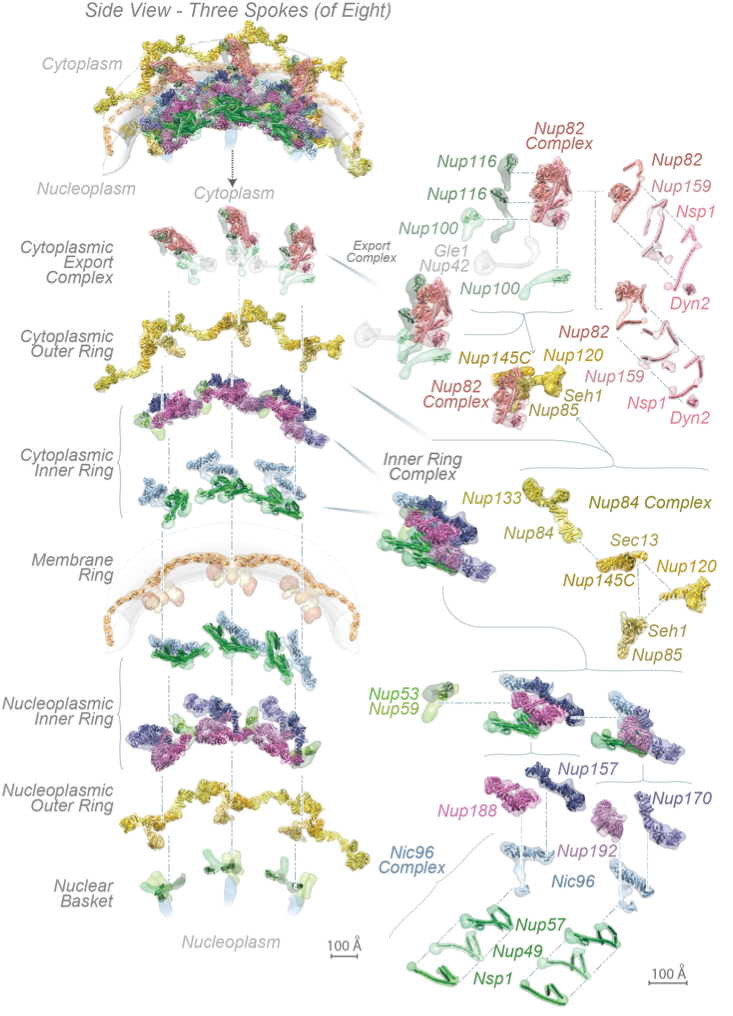
The complete structure of the NPC and its components. For each Nup, the localization probability density of the ensemble of structures is shown with a representative structure from the ensemble embedded within it. (Top) Three consecutive NPC spokes,showing how the coaxial outer, inner, and membrane rings run continuously between spokes. (Bottom) Relative position of major NPC components and connections both within and between spokes, plus exploded view of three consecutive spokes and their complexes.
These Nups assemble into sub-complexes that form higher-order structures called spokes. Eight spokes assemble into even larger, discrete and relatively rigid modules: coaxial outer and inner rings form a symmetric core scaffold at the heart of which are sturdy diagonal columns. This scaffold is connected to a membrane ring and two peripheral assemblies, a nuclear basket and a cytoplasmic RNP export platform, all of which are. Remarkably, flexible connectors run the entire length of each spoke, tying together every major element in the NPC.
They link the periphery and outer rings to the inner rings, both inner rings to the pore membrane and adjacent spokes to one another. This combination of rigid modules held together by flexible connectors appears to imbue the structure with both strength and flexibility, being reminiscent of a suspension bridge, in which rigid supporting columns are firmly anchored to a substrate and flexible suspension cables connect the columns and roadway to provide a strong and resilient structure. The scaffold surrounds a central channel that is formed in part by nucleoporins termed FG Nups, from which multiple intrinsically disordered Phe-Gly (FG) repeat motifs project, contributing to a central density termed the central transporter. These FG motifs mediate selective nucleocytoplasmic transport through specific interactions with nuclear transport factors. The structured regions of the NPC largely direct the FG-repeat regions inwards toward the axis of the central channel, instead of projecting from the NPC towards the cytoplasm and nucleoplasm as they are often represented. This geometry generates a highly concentrated and dynamic FG-repeat phase through which cargo-carrying transport factors readily pass, facilitated by their specific FG interactions, whereas nonspecific macromolecular diffusion is hindered by this same dense phase. Our map also shows that the entire export platform positions the cytoplasmic mRNA export factor docking sites and messenger ribonucleoprotein (mRNP) remodeling machinery right over the NPC’s central channel rather than on distal cytoplasmic filaments, as previously supposed. We suggest that this configuration efficiently captures and remodels exporting mRNP particles immediately upon reaching the cytoplasmic side of the NPC.
Most recently, we generated a structure of the isolated yeast NPC in which the inner ring is resolved by cryo-EM at sub-nanometer resolution, to show how flexible connectors tie together different structural and functional layers. These connectors are extremely dynamic, able to reversibly dissociate from the otherwise stably-associated NPC modules they interconnect, helping to provide the NPC’s overall structure with both strength and flexibility. Moreover, these connectors may be targets for phosphorylation and regulated disassembly in cells with an open mitosis. Intriguingly, some nucleoporin and transport factor pairs have similar interaction motifs, which suggests an evolutionary and mechanistic link between assembly and transport. We also provided evidence for three major NPC variants that may foreshadow functional specializations at the nuclear periphery. Cryo-electron tomography extended these studies, providing a model of the in situ NPC with a radially-expanded inner ring. Our comprehensive model reveals features of the nuclear basket and central transporter, suggesting a role for the lumenal Pom152 ring in restricting dilation and highlighting the NPC’s structural plasticity, that may be required for transport.
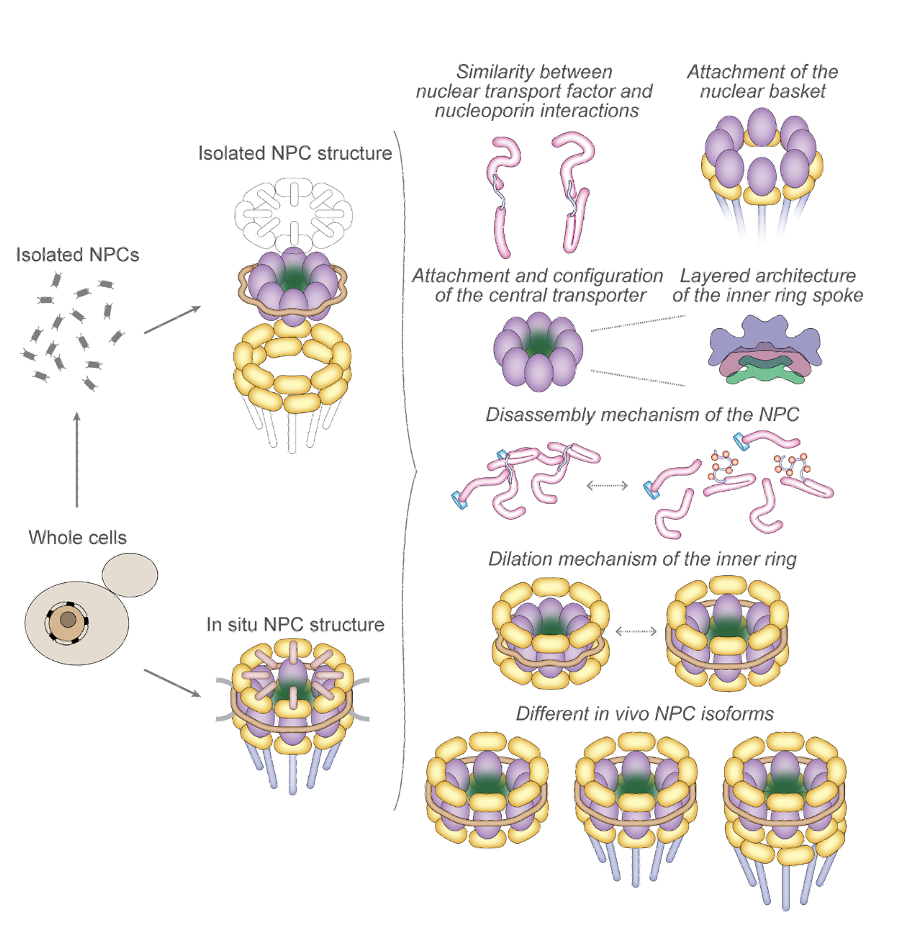
Diagram summarizing main findings from the latest NPC structures.
Cryo-EM data from both isolated and in situ yeast NPCs (left) were combined with crosslinking-mass spectrometry data to generate a comprehensive map of the yeast NPC. The main functional findings from this map are summarized at right.
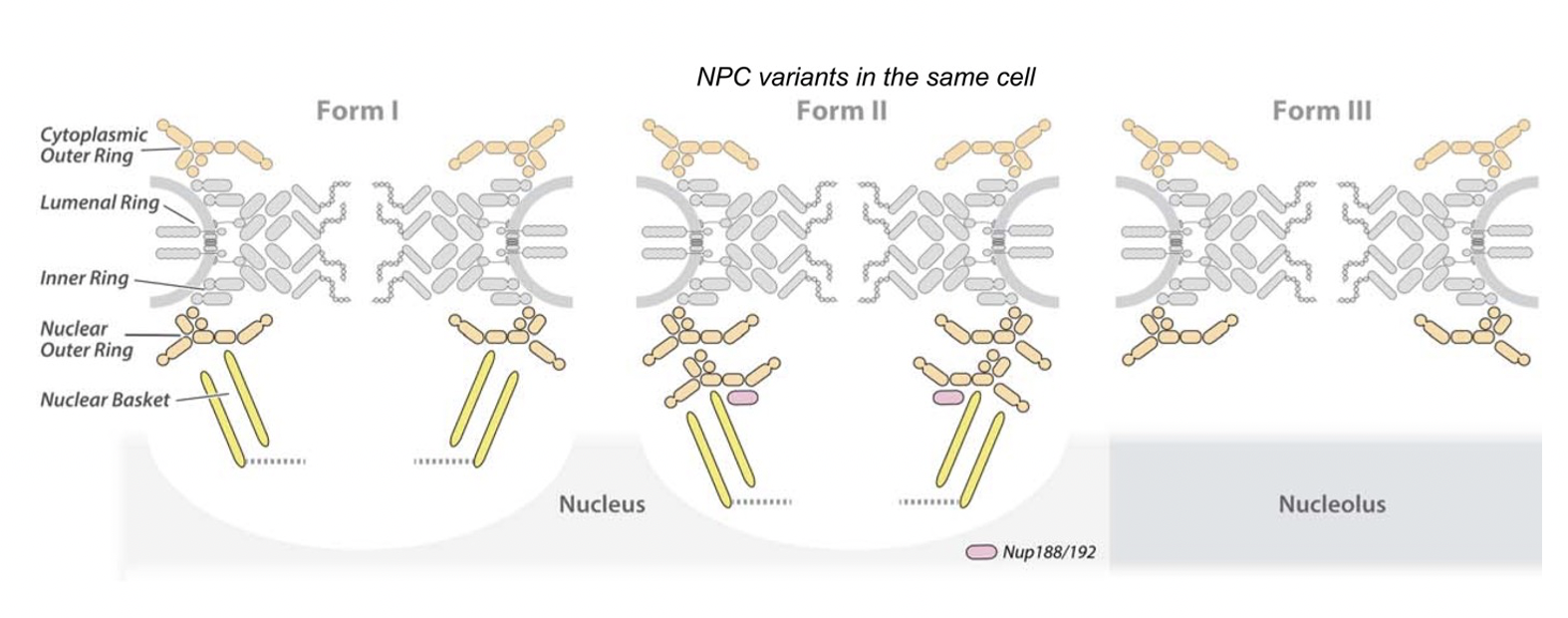
Diagram summarizing the salient differences between the three NPC isoforms found in yeast cells.
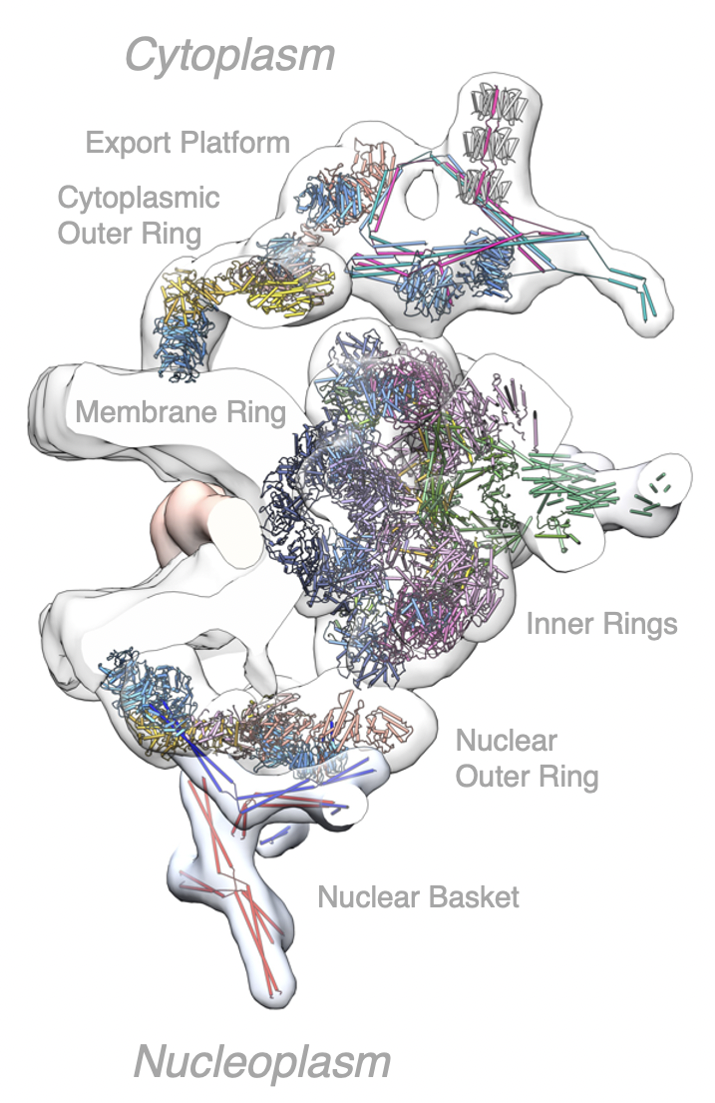
Structure of one spoke from the yeast NPC
The export platform can be seen to project inwards to the central transport axis of the NPC.
We are also dissecting the NPC architecture of a highly divergent eukaryote, Trypanosoma brucei in order comparing it to the available structures from yeast and humans, to unravel the molecular processes that governed the evolution and re-purposing of membrane-coating complexes during the origin of the nucleus, and to provide orthogonal insights into what are the most essential features of the nucleocytoplasmic transport machinery. Moreover, we are studying NPCs in human cells to determine how alterations in the NPC during oncogenesis and viral infection can lead to disease.
References:
Rout et al., 2000 (PDF)
Devos et al., 2004 (PDF) -missing
Devos et al., 2006 (PDF)
Alber et al, 2007 A (PDF)
Fernandez-Martinez et at., 2016 (PDF) -bad file
Kim et al., 2018 (PDF)
Hakhverdyan et at., 2020 (PDF)
Fernandez-Martinez et at., 2021 (PDF)
Akey et at._ 2022
This work is an ongoing collaboration with
the Aitchison, Akey, Chait, Fernandez-Martinez, Ludtke, Sali, and Villa laboratories.