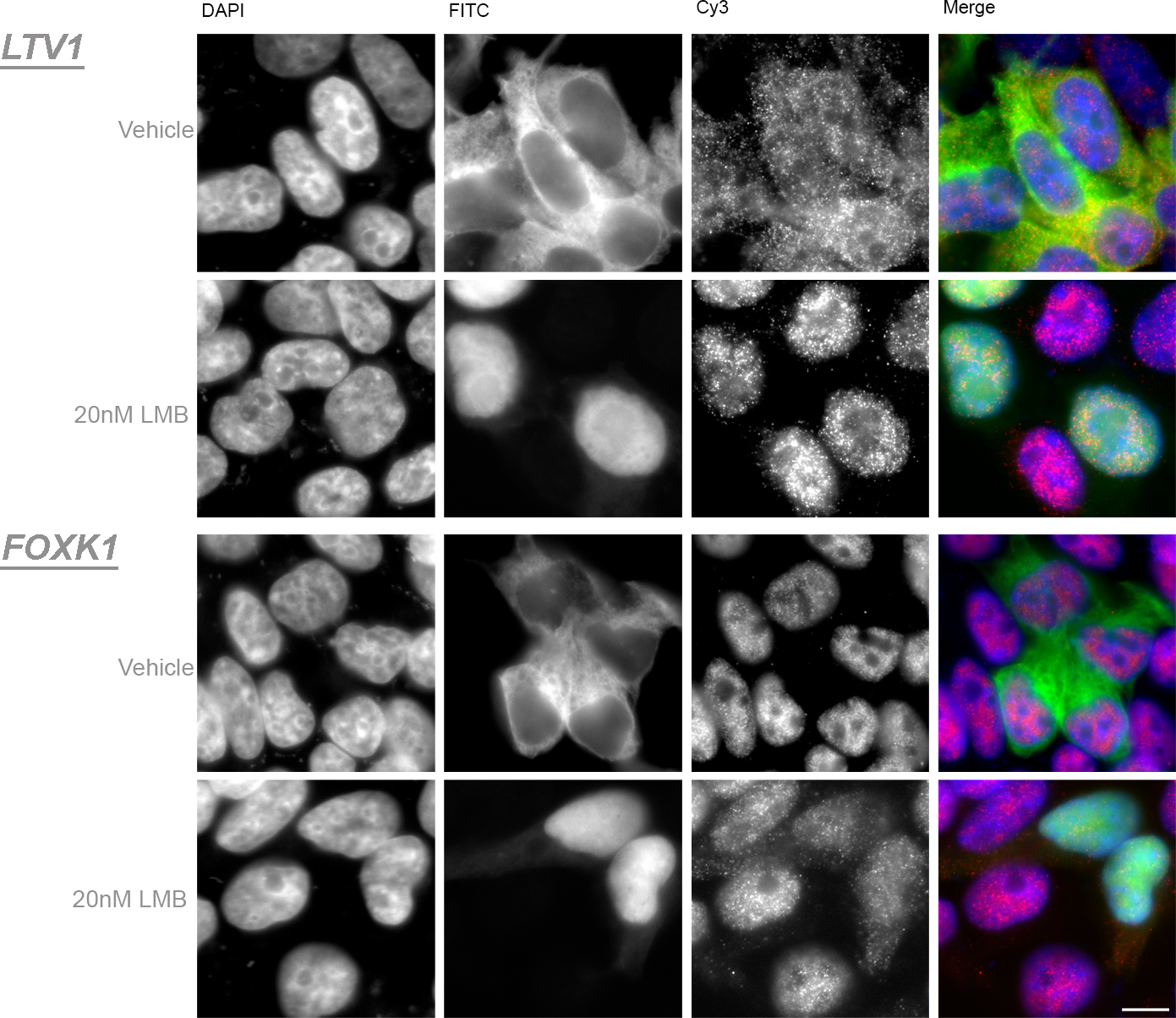
Nuclear Transport and Disease
The NPC and Cancers: An Emerging Drug Target
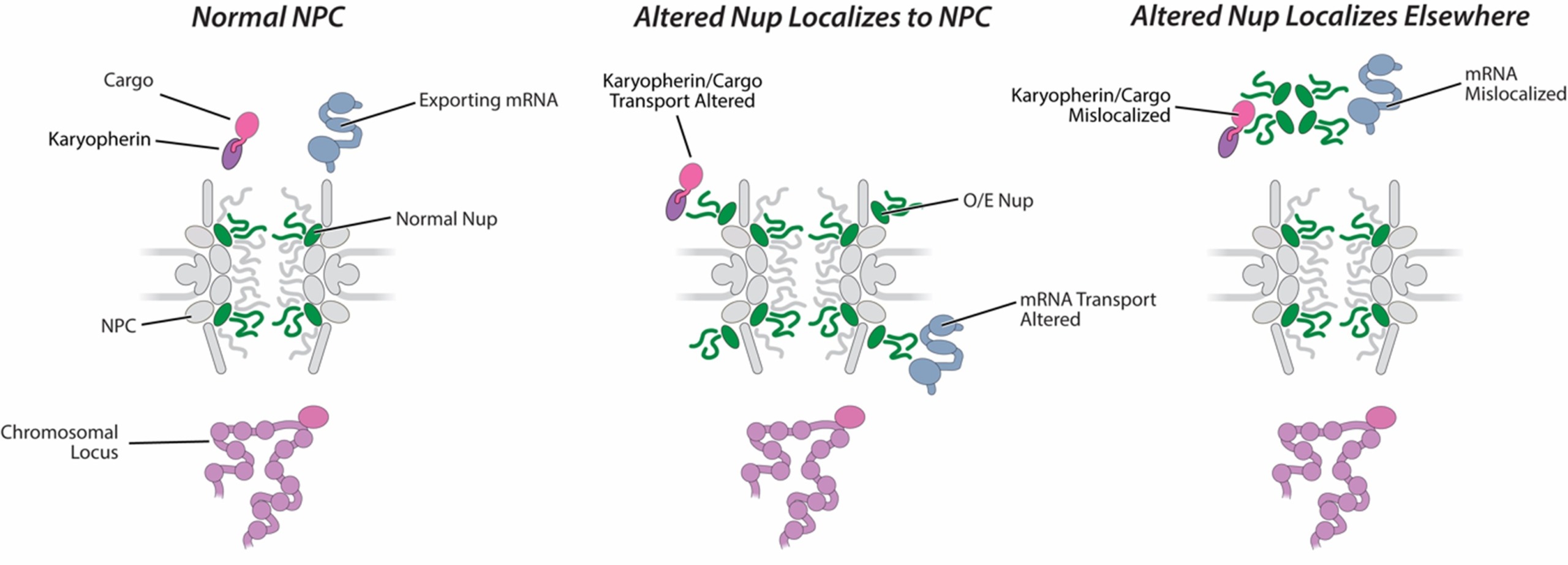
A specific subset of Nups has been linked to human cancers. The most strongly linked are TPR, Nup62, and the cytoplasmic constituents Nup88, Nup98, Nup214, and Nup358/RanBP2. These Nups are implicated in a wide variety of cancers via three mutational routes, namely: chromosomal translocations; single point mutations; and changes in Nup expression levels. The most prevalent types of NPC-related oncogenic mutations are chromosomal translocations that fuse portions of a Nup gene with another gene, generating chimeric fusions with novel functions. Rather than fusions, many tumors instead show greatly increased expression levels of Nup62 and Nup88. The fact that these various oncogenic states have Nup defects in common, despite different underlying causes (fusion vs overexpression) underscores how alteration of NPC function is a key step towards oncogenesis.
The mechanisms by which Nup overexpression or Nup fusion mutations lead to cancer are mostly unknown. Indeed, even the localizations of many of the fusion proteins have not been determined, although at least some may localize to the NPC. Nevertheless, either by direct association with the NPC or by mislocalization of critical components, there is strong evidence that these Nup alterations misregulate nucleocytoplasmic transport and communication, which may aberrantly affect gene transcription and signaling pathways. Many viruses also target the NPC by altering either the structure, copy number, or phosphorylation state of specific Nups. Significantly, the Nups they target are the same ones as are associated with cancers. As viruses have been termed “nature’s cell biologists,” it is likely that they are exploiting some of the same weaknesses as the oncogenic defects to alter cell behaviour and growth for their own gain, further bolstering the idea that alterations of specific Nups underlie the mechanisms of disease.
Nuclear – Mitochondria Communication in Cancer
The nucleus and the mitochondria are the two main genome-bearing organelles with key roles in cellular and organismal physiology. The cross talk between them plays a pivotal role in maintenance of mitochondrial biogenesis and functionality during stress and aging. Various signals trigger interorganellar compensatory responses between the nucleus and the mitochondria. These responses are highly orchestrated and any misregulation or imbalance can result in loss of cellular homeostasis. A major causative connection in cancer and the NPC is the communication between the nucleus and the mitochondria, in the apoptotic signaling pathway. Crm1 (Exportin 1 or XPO1) is the major nuclear exporter of tumor suppressor proteins, oncoproteins and growth regulatory proteins including p53, Survivin, FOXO, p21, p73, BRCA1 and many more. Their relative abundances in the nucleus and cytoplasm are controlled through regulated nucleocytoplasmic transport shuttling. Elevated nuclear export processes have been found in a broad spectrum of cancers as well as upregulated levels of Crm1. Many of these proteins normally localize mainly in the nucleus, where they function as transcription regulators and aid in chromosomal assembly. Improper localization may lead to inactivation as tumor suppressors or excess of anti-apoptotic activity of oncoproteins. Importantly, several Crm1 cargos can also be found in the mitochondria once they are exported out of the nucleus, for example p53, BRCA1 and Bok). BRCA1 function in the mitochondria is still unknown, but p53 and Bok are known to be key players in the intrinsic apoptotic pathway governed by the mitochondria. The intrinsic apoptotic pathway is triggered by intracellular damage or by oncogenic stress. This process is regulated and controlled by the Bcl-2 family proteins while p53 play a critical role in their regulation. All these reports together with numerous studies indicating induced apoptosis with the inhibition of nuclear export point to extensive interplay between the nucleus and the mitochondrial apoptotic pathway. since all import and export pathways converge at the NPC, Nups may be another potentially powerful set of therapeutic targets – if we were to better understand the oncogenic mechanism.
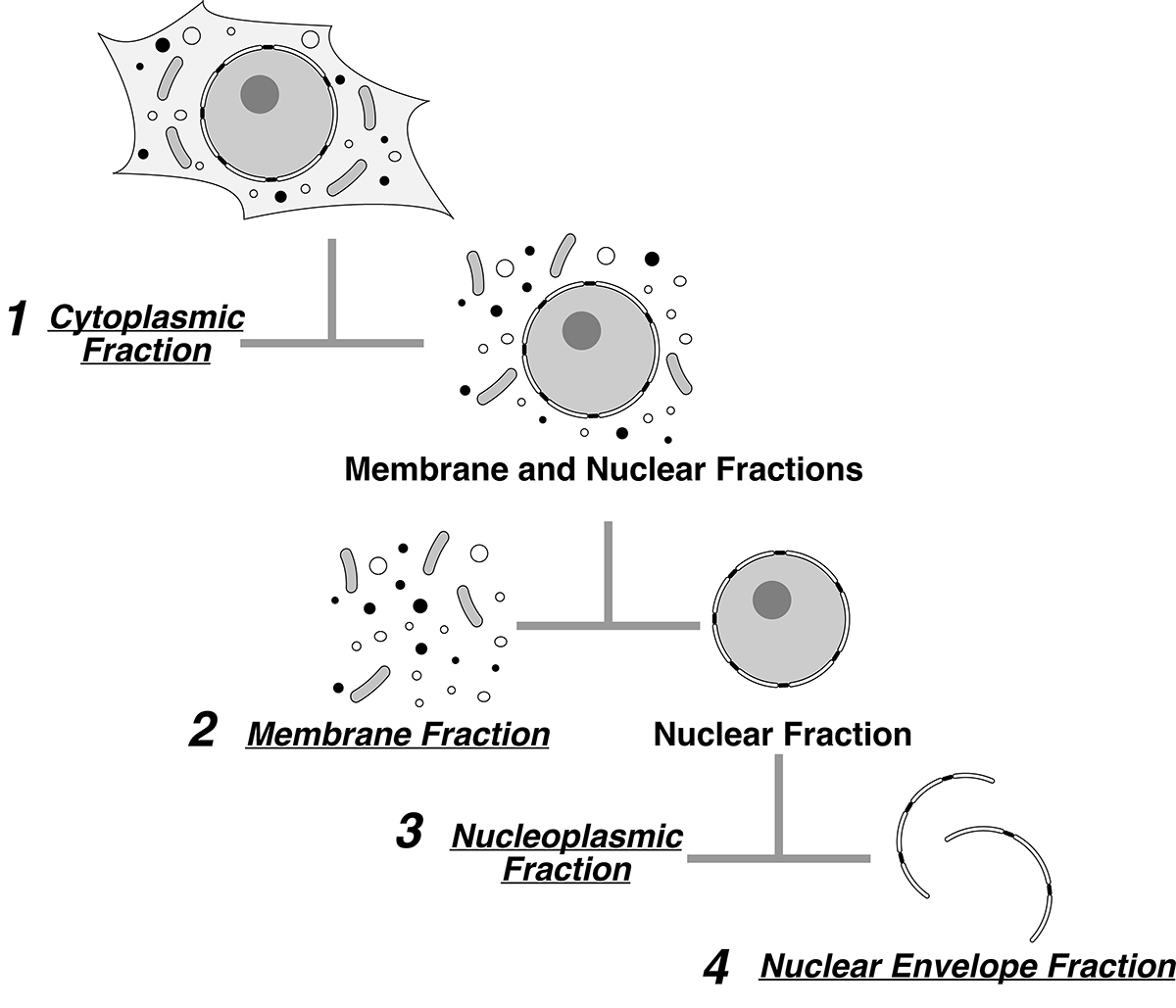
In order to study the distribution of key players during oncogenesis we have developed a novel quantitative cellular fractionation method for mammalian cells. Subcellular fractionation in combination with mass spectrometry-based proteomics is a powerful tool to study localization of key proteins in health and disease. We developed a reliable and rapid method for mammalian cell fractionation, tuned for such proteomic analyses. This method is applicable to different cell lines in which all the cellular contents are accounted for, while maintaining nuclear and nuclear envelope integrity. This method provides a fast and reliable means to perform mass spectrometry and DeDuvian enrichment analyses for the different cellular compartments.